
HL Paper 2
Two hydrides of nitrogen are ammonia and hydrazine, . One derivative of ammonia is methanamine whose molecular structure is shown below.

Hydrazine is used to remove oxygen from water used to generate steam or hot water.
The concentration of dissolved oxygen in a sample of water is .
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
State the electron domain geometry around the nitrogen atom and its hybridization in methanamine.
Ammonia reacts reversibly with water.
Explain the effect of adding ions on the position of the equilibrium.
Hydrazine reacts with water in a similar way to ammonia. (The association of a molecule of hydrazine with a second H+ is so small it can be neglected.)
Calculate the pH of a solution of hydrazine.
Suggest a suitable indicator for the titration of hydrazine solution with dilute sulfuric acid using section 22 of the data booklet.
Outline, using an ionic equation, what is observed when magnesium powder is added to a solution of ammonium chloride.
Determine the enthalpy change of reaction, , in kJ, when 1.00 mol of gaseous hydrazine decomposes to its elements. Use bond enthalpy values in section 11 of the data booklet.
The standard enthalpy of formation of is . Calculate the enthalpy of vaporization, , of hydrazine in . (If you did not get an answer to (f), use but this is not the correct answer.)
Calculate, showing your working, the mass of hydrazine needed to remove all the dissolved oxygen from of the sample.
Calculate the volume, in , of nitrogen formed under SATP conditions. (The volume of 1 mol of gas = at SATP.)
Markscheme
107°
Accept 100° to < 109.5°.
Literature value = 105.8°
[1 mark]
tetrahedral
sp3
No ECF allowed.
[2 marks]
removes/reacts with
moves to the right/products «to replace ions»
Accept ionic equation for M1.
[2 marks]
Kb = 10–5.77 / 1.698 x 10–6
OR
[OH–]2 «= 1.698 × 10–6 × 0.0100» = 1.698 × 10–8
OR
[OH–] «» = 1.303 × 10–4 «mol dm–3»
pH «» = 10.1
Award [3] for correct final answer.
Give appropriate credit for other methods containing errors that do not yield correct final answer.
[3 marks]
methyl red
OR
bromocresol green
OR
bromophenol blue
OR
methyl orange
[1 mark]
bubbles
OR
gas
OR
magnesium disappears
Do not accept “hydrogen” without reference to observed changes.
Accept "smell of ammonia".
Accept 2H+(aq) + Mg(s) Mg2+(aq) + H2(g)
Equation must be ionic.
[2 marks]
bonds broken:
E(N–N) + 4E(N–H)
OR
bonds formed:
E(NN) + 2E(H–H)
OR
Award [3] for correct final answer.
Award [2 max] for +95 «kJ».
[3 marks]

OR
Award [2] for correct final answer. Award [1 max] for –44 «kJ mol–1».
Award [2] for:
ΔHvap = –50.6 kJ mol–1 – (–85 J mol–1) = +34 «kJ mol–1».
Award [1 max] for –34 «kJ mol–1».
[2 marks]
total mass of oxygen
OR
Award [3] for correct final answer.
[3 marks]
Award [1] for correct final answer.
[1 mark]
Examiners report
Lewis (electron dot) structures are useful models.
Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory to deduce the molecular geometry of each species including bond angles.
Predict whether the molecules PF3 and PF5 are polar or non-polar.
State the type of hybridization shown by the phosphorus atom in PF3.
Markscheme
Accept any combination of dots, crosses and lines.
Penalize missing lone pairs once only.
Do not apply ECF for molecular geometry.
Accept values in the range 95–109 for PF3.
PF3 polar AND PF5 non-polar
Apply ECF from part (a) molecular geometry.
sp3
Examiners report
Bonds can be formed in many ways.
The equilibrium for a mixture of NO2 and N2O4 gases is represented as:
2NO2(g) N2O4(g)
At 100°C, the equilibrium constant, Kc, is 0.21.
Bonds can be formed in many ways.
Discuss the bonding in the resonance structures of ozone.
Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen atom.
The first six ionization energies, in kJ mol–1, of an element are given below.

Explain the large increase in ionization energy from IE3 to IE4.
At a given time, the concentration of NO2(g) and N2O4(g) were 0.52 and respectively.
Deduce, showing your reasoning, if the forward or the reverse reaction is favoured at this time.
Comment on the value of ΔG when the reaction quotient equals the equilibrium constant, Q = K.
Markscheme
lone pair on p orbital «of O atom» overlaps/delocalizes with pi electrons «from double bond»
both O–O bonds have equal bond length
OR
both O–O bonds have same/1.5 bond order
OR
both O–O are intermediate between O–O AND O=O
both O–O bonds have equal bond energy
Accept “p/pi/ electrons are delocalized/not localized”.
[3 marks]
ALTERNATIVE 1:
FC: –1 AND +1 AND 0
ALTERNATIVE 2:
FC: 0 AND +1 AND –1
Accept any combination of lines, dots or crosses to represent electrons.
Do not accept structure that represents 1.5 bonds.
Do not penalize missing lone pairs if already penalized in 3(b).
If resonance structure is incorrect, no ECF.
Any one of the structures with correct formal charges for [2 max].
[2 marks]
Any two of:
IE4: electron in lower/inner shell/energy level
OR
IE4: more stable/full electron shell
IE4: electron closer to nucleus
OR
IE4: electron more tightly held by nucleus
IE4: less shielding by complete inner shells
Accept “increase in effective nuclear charge” for M2.
[2 marks]
«Qc = =» 0.37
reaction proceeds to the left/NO2(g) «until Q = Kc»
OR
reverse reaction «favoured»
Do not award M2 without a calculation for M1 but remember to apply ECF.
[2 marks]
ΔG = 0
reaction at equilibrium
OR
rate of forward and reverse reaction is the same
OR
constant macroscopic properties
[2 marks]
Examiners report
Organic chemistry can be used to synthesize a variety of products.
Combustion analysis of an unknown organic compound indicated that it contained only carbon, hydrogen and oxygen.
Several compounds can be synthesized from but-2-ene. Draw the structure of the final product for each of the following chemical reactions.
Determine the change in enthalpy, ΔH, for the combustion of but-2-ene, using section 11 of the data booklet.
CH3CH=CHCH3 (g) + 6O2 (g) → 4CO2 (g) + 4H2O (g)
State the hybridization of the carbon I and II atoms in but-2-ene.
Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms.
Sketch the mechanism for the reaction of 2-methylbut-2-ene with hydrogen bromide using curly arrows.
Explain why the major organic product is 2-bromo-2-methylbutane and not 2-bromo-3-methylbutane.
Deduce two features of this molecule that can be obtained from the mass spectrum. Use section 28 of the data booklet.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce
on behalf of the United States of America. All rights reserved.
Identify the bond responsible for the absorption at A in the infrared spectrum. Use section 26 of the data booklet.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce
on behalf of the United States of America. All rights reserved.
Deduce the identity of the unknown compound using the previous information, the 1H NMR spectrum and section 27 of the data booklet.
SDBS, National Institute of Advanced Industrial Science and Technology (AIST).
Draw the stereoisomers of butan-2-ol using wedge-dash type representations.
Outline how two enantiomers can be distinguished using a polarimeter.
Markscheme
Penalize missing hydrogens in displayed structural formulas once only.
Accept condensed structural formulas: CH3CH(OH)CH2CH3 / CH3CH2CH2CH3 or skeletal structures.
Bonds broken:
2(C–C) + 1(C=C) + 8(C–H) + 6O=O / 2(346) + 1(614) + 8(414) + 6(498) / 7606 «kJ» ✓
Bonds formed:
8(C=O) + 8(O–H) / 8(804) + 8(463) / 10 136 «kJ» ✓
Enthalpy change:
«Bonds broken – Bonds formed = 7606 kJ – 10 136 kJ =» –2530 «kJ» ✓
Award [3] for correct final answer.
Award [2 max] for «+» 2530 «kJ».
Sigma (σ):
Accept any diagram showing end to end/direct overlap of atomic/hybridized orbitals and electron density concentrated between nuclei.
Pi (π):
Accept any diagram showing sideways overlap of unhybridized p/atomic orbitals and electron density above and below plane of bond axis.
Alternative 1
Penalize incorrect bond e.g., -CH-H3C or –CH3C only once in the paper.
Alternative 2
curly arrow going from C=C to H of HBr AND curly arrow showing Br leaving ✓
representation of carbocation ✓
curly arrow going from lone pair/negative charge on Br− to C+ ✓
«2-bromo-2-methylbutane involves» formation of more stable «tertiary» carbocation/intermediate
OR
«2-bromo-3-methylbutane involves» formation of less stable «secondary» carbocation/intermediate ✓
«intermediate» more stable due to «increased positive» inductive/electron-releasing effect of extra –R/alkyl group/–CH3/methyl ✓
Do not award marks for quoting Markovnikov’s rule without any explanation.
m/z 58:
molar/«relative» molecular mass/weight/Mr «is 58 g mol−1/58» ✓
m/z 43:
«loses» methyl/CH3 «fragment»
OR
COCH3+ «fragment» ✓
Do not penalize missing charge on the fragments.
Accept molecular ion «peak»/ CH3COCH3+/C3H6O+.
Accept any C2H3O+ fragment/ CH3CH2CH2+/C3H7+.
C=O ✓
Accept carbonyl/C=C.
Information deduced from 1H NMR:
«one signal indicates» one hydrogen environment/symmetrical structure
OR
«chemical shift of 2.2 indicates» H on C next to carbonyl ✓
Compound:
propanone/CH3COCH3 ✓
Accept “one type of hydrogen”.
Accept .
enantiomers rotate «plane of» plane-polarized light ✓
equal degrees/angles/amounts AND opposite directions/rotation ✓
Accept “optical isomers” for “enantiomers”.
Examiners report
Compound A is in equilibrium with compound B.
Predict the electron domain and molecular geometries around the oxygen atom of molecule A using VSEPR
State the type of hybridization shown by the central carbon atom in molecule B.
State the number of sigma () and pi () bonds around the central carbon atom in molecule B.
The IR spectrum of one of the compounds is shown:
COBLENTZ SOCIETY. Collection © 2018 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Deduce, giving a reason, the compound producing this spectrum.
Compound A and B are isomers. Draw two other structural isomers with the formula .
The equilibrium constant, , for the conversion of A to B is in water at .
Deduce, giving a reason, which compound, A or B, is present in greater concentration when equilibrium is reached.
Calculate the standard Gibbs free energy change, , in , for the reaction (A to B) at . Use sections 1 and 2 of the data booklet.
Propanone can be synthesized in two steps from propene. Suggest the synthetic route including all the necessary reactants and steps.
Propanone can be synthesized in two steps from propene.
Suggest why propanal is a minor product obtained from the synthetic route in (g)(i).
Markscheme
Electron domain geometry: tetrahedral ✔
Molecular geometry: bent/V-shaped ✔
✔
-bonds:
AND
-bonds: ✔
B AND absorption/
OR
B AND absence of ✔
Accept any value between .
Accept any two isomers except for propanone and propen-2-ol:
✔✔
Penalize missing hydrogens in displayed structural formulas once only.
AND is greater than /large ✔
✔
/water «and » ✔
/propan-2-ol ✔
/«potassium» dichromate(VI) AND
OR
/«acidified potassium» manganate(VII) ✔
Accept .
primary carbocation «intermediate forms»
OR
minor product «of the water addition would be» propan-1-ol
OR
anti-Markovnikov addition of water ✔
primary alcohol/propan-1-ol oxidizes to an aldehyde/propanal ✔
Examiners report
The majority of students got at least one of electron domain geometry or molecular geometry correct.
The vast majority of students could identify the hybridization around a central carbon atom.
The vast majority of students could identify BOTH sigma and pi bonds in a molecule.
Many candidates identified B having C = O and a peak at 1750.
A surprising number of candidates drew propanone here as an option, either failing to read the question or perhaps finding the structural formulae provided difficult to understand.
Most candidates identified B, the product, as being in greater concentration at equilibrium however some lost the mark because they did not include a reason.
Most candidates could apply the formula for Gibbs free energy change, ΔGΘ, correctly however some did not get the units correct.
The mean mark was ⅔ for the required synthetic route. Some candidates failed to identify water as a reagent in the hydration reaction, or note that dichromate ion oxidation requires acidic conditions. This was also the question with most No Response.
This question regarding the formation of a minor product was not well answered. Many candidates struggled to explain the formation of propan-1-ol and to then oxidize it to propanal.
Some physical properties of molecular substances result from the different types of forces between their molecules.
Resonance structures exist when a molecule can be represented by more than one Lewis structure.
Carbon dioxide can be represented by at least two resonance structures, I and II.

Calculate the formal charge on each oxygen atom in the two structures.

Deduce, giving a reason, the more likely structure.
Absorption of UV light in the ozone layer causes the dissociation of oxygen and ozone.
Identify, in terms of bonding, the molecule that requires a longer wavelength to dissociate.
Carbon and silicon are elements in group 14.
Explain why CO2 is a gas but SiO2 is a solid at room temperature.
Markscheme

Award [1] for any two correctly filled cells.
[2 marks]
structure I AND no formal charges
OR
structure I AND no charge transfer «between atoms»
[1 mark]
O3 has bond between single and double bond AND O2 has double bond
OR
O3 has bond order of 1.5 AND O2 has bond order of 2
OR
bond in O3 is weaker/longer than in O2
O3 requires longer wavelength
M1: Do not accept “ozone has one single and one double bond”.
[2 marks]
CO2 «non-polar» «weak» London/dispersion forces/instantaneous induced dipole-induced dipole forces between molecules
SiO2 network/lattice/3D/giant «covalent» structure
M1: The concept of “between” is essential.
[2 marks]
Examiners report
Butanoic acid, CH3CH2CH2COOH, is a weak acid and ethylamine, CH3CH2NH2, is a weak base.
State the equation for the reaction of each substance with water.
Draw a diagram showing the delocalization of electrons in the conjugate base of butanoic acid.
Deduce the average oxidation state of carbon in butanoic acid.
A 0.250 mol dm−3 aqueous solution of butanoic acid has a concentration of hydrogen ions, [H+], of 0.00192 mol dm−3. Calculate the concentration of hydroxide ions, [OH−], in the solution at 298 K.
Determine the pH of a 0.250 mol dm−3 aqueous solution of ethylamine at 298 K, using section 21 of the data booklet.
Sketch the pH curve for the titration of 25.0 cm3 of ethylamine aqueous solution with 50.0 cm3 of butanoic acid aqueous solution of equal concentration. No calculations are required.
Explain why butanoic acid is a liquid at room temperature while ethylamine is a gas at room temperature.
State a suitable reagent for the reduction of butanoic acid.
Deduce the product of the complete reduction reaction in (e)(i).
Markscheme
Butanoic acid:
CH3CH2CH2COOH (aq) + H2O (l) CH3CH2CH2COO− (aq) + H3O+ (aq) ✔
Ethylamine:
CH3CH2NH2 (aq) + H2O (l) CH3CH2NH3+ (aq) + OH− (aq) ✔
Diagram showing:
dotted line along O–C–O AND negative charge
Accept correct diagrams with pi clouds.
–1 ✔
«» = 5.21 × 10–12 «mol dm–3» ✔
«pKb = 3.35, Kb = 10–3.35 = 4.5 × 10–4»
«C2H5NH2 + H2O C2H5NH3+ + OH–»
Kb =
OR
«Kb =» 4.5 × 10–4 =
OR
«Kb =» 4.5 × 10–4 = ✔
« x = [OH–] =» 0.011 «mol dm–3» ✔
«pH = –log» 12.04
OR
«pH = 14.00 – (–log 0.011)=» 12.04 ✔
Award [3] for correct final answer.
decreasing pH curve ✔
pH close to 7 (6–8) at volume of 25 cm3 butanoic acid ✔
weak acid/base shape with no flat «strong acid/base» parts on the curve ✔
Any two of:
butanoic acid forms more/stronger hydrogen bonds ✔
butanoic acid forms stronger London/dispersion forces ✔
butanoic acid forms stronger dipole–dipole interaction/force ✔
Accept “butanoic acid forms dimers”
Accept “butanoic acid has larger Mr/hydrocarbon chain/number of electrons” for M2.
Accept “butanoic acid has larger «permanent» dipole/more polar” for M3.
lithium aluminium hydride/LiAlH4 ✔
butan-1-ol/1-butanol/CH3CH2CH2CH2OH ✔
Examiners report
The overall equation for the production of hydrogen cyanide, HCN, is shown below.
CH4 (g) + NH3 (g) +O2 (g) → HCN (g) + 3H2O (g)
State why NH3 is a Lewis base.
Calculate the pH of a 1.00 × 10−2 mol dm−3 aqueous solution of ammonia.
pKb = 4.75 at 298 K.
Justify whether a 1.0 dm3 solution made from 0.10 mol NH3 and 0.20 mol HCl will form a buffer solution.
Sketch the shape of one sigma () and one pi () bond.
Identify the number of sigma and pi bonds in HCN.
State the hybridization of the carbon atom in HCN.
Suggest why hydrogen chloride, HCl, has a lower boiling point than hydrogen cyanide, HCN.
Explain why transition metal cyanide complexes are coloured.
Markscheme
donates «lone/non-bonding» pair of electrons ✔
Kb = 10-4.75 /1.78 x 10-5
OR
Kb = ✔
[OH–] = « =» 4.22 × 10–4 «(mol dm–3)» ✔
pOH« = –log10 (4.22 × 10–4)» = 3.37
AND
pH = «14 – 3.37» = 10.6
OR
[H+]« =» = 2.37 × 10–11
AND
pH« = –log10 2.37 × 10–11» = 10.6 ✔
Award [3] for correct final answer.
no AND is not a weak acid conjugate base system
OR
no AND weak base «totally» neutralized/ weak base is not in excess
OR
no AND will not neutralize small amount of acid ✔
Accept “no AND contains 0.10 mol NH4Cl + 0.10 mol HCl”.
Sigma ():
Pi ():
Accept overlapping p-orbital(s) with both lobes of equal size/shape.
Shaded areas are not required in either diagram.
Sigma (): 2 AND Pi (): 2 ✔
sp ✔
HCN has stronger dipole–dipole attraction ✔
Do not accept reference to H-bonds.
Any three from:
partially filled d-orbitals ✔
«CN- causes» d-orbitals «to» split ✔
light is absorbed as electrons transit to a higher energy level «in d–d transitions»
OR
light is absorbed as electrons are promoted ✔
energy gap corresponds to light in the visible region of the spectrum ✔
Do not accept “colour observed is the complementary colour” for M4.
Examiners report
The main error was the omission of lone electron "pair", though there was also a worrying amount of very confused answers for a very basic chemistry concept where 40% provided very incorrect answers.
Rather surprisingly, many students got full marks for this multi-step calculation; others went straight to the pH/pKa acid/base equation so lost at least one of the marks: students often seem less prepared for base calculations, as opposed to acid calculations.
Poorly answered revealing little understanding of buffering mechanisms, which is admittedly a difficult topic.
This proved to be the most challenging question (10%). It was a good question, where candidates had to explain a huge difference in boiling point of two covalent compounds, requiring solid understanding of change of state where breaking bonds cannot be involved). Yet most considered the triple bonds in HCN as the cause, suggesting covalent bonds break when substance boil, which is very worrying. Others considered H-bonds which at least is an intermolecular force, but shows they are not too familiar with the conditions necessary for H-bonding.
This question appears frequently in exams but with slightly different approaches. In general candidates ignore the specific question and give the same answers, failing in this case to describe why complexes are coloured rather than what colour is seen. These answers appear to reveal that many candidates don't really understand this phenomenon, but learn the answer by heart and make mistakes when repeating it, for example, stating that the ‘d-orbitals of the ligands were split’- an obvious misconception. The average mark was 1.6/3, with a MS providing 4 ideas that would merit a mark
There is concern about damage done to the ozone layer in the stratosphere by jet-propelled aircraft.
Formulate two equations to show how nitrogen(II) oxide, NO, catalyses the destruction of ozone.
Suggest why the loss of ozone is an international environmental concern.
Markscheme
OR
Allow representation of radicals without if consistent throughout.
[2 marks]
«loss of ozone» allows UV radiation to penetrate atmosphere/reach earth
UV radiation causes skin cancer
OR
UV radiation causes tissue damage
[2 marks]
Examiners report
Nitric acid is usually produced by the oxidation of ammonia.
A mixture of nitric acid and sulfuric acid can be used to convert benzene to nitrobenzene, C6H5NO2.
Draw arrows in the boxes to represent the electron configuration of a nitrogen atom.
Deduce a Lewis (electron dot) structure of the nitric acid molecule, HNO3, that obeys the octet rule, showing any non-zero formal charges on the atoms.
Explain the relative lengths of the three bonds between N and O in nitric acid.
State a technique used to determine the length of the bonds between N and O in solid HNO3.
Write an equation for the reaction between the acids to produce the electrophile, NO2+.
Draw the structural formula of the carbocation intermediate produced when this electrophile attacks benzene.
Deduce the number of signals that you would expect in the 1H NMR spectrum of nitrobenzene and the relative areas of these.
Markscheme
Accept all 2p electrons pointing downwards.
Accept half arrows instead of full arrows.
bonds and non-bonding pairs correct ✔
formal charges correct ✔
Accept dots, crosses or lines to represent electron pairs.
Do not accept resonance structures with delocalised bonds/electrons.
Accept + and – sign respectively.
Do not accept a bond between nitrogen and hydrogen.
For an incorrect Lewis structure, allow ECF for non-zero formal charges.
Any three of:
two N-O same length/order ✔
delocalization/resonance ✔
N-OH longer «than N-O»
OR
N-OH bond order 1 AND N-O bond order 1½ ✔
Award [2 max] if bond strength, rather than bond length discussed.
Accept N-O between single and double bond AND N-OH single bond.
X-ray crystallography ✔
HNO3 + 2H2SO4 NO2+ + H3O+ + 2HSO4- ✔
Accept “HNO3 + H2SO4 NO2+ + H2O + HSO4-”.
Accept “HNO3 + H2SO4 H2NO3+ + HSO4-” AND “H2NO3+ NO2+ + H2O”.
Accept single arrows instead of equilibrium signs.
Accept any of the five structures.
Do not accept structures missing the positive charge.
Number of signals: three/3 ✔
Relative areas: 2 : 2 : 1 ✔
Examiners report
Drawing arrows in the boxes to represent the electron configuration of a nitrogen atom was done extremely well.
Drawing the Lewis structure of HNO3 was performed extremely poorly with structures that included H bonded to N, no double bond or a combination of single, double and even a triple bond or incorrect structures with dotted lines to reflect resonance. Many did not calculate non-zero formal charges.
Poorly done; some explained relative bond strengths between N and O in HNO3, not relative lengths; others included generic answers such as triple bond is shortest, double bond is longer, single longest.
A majority could not state the technique to determine length of bonds; answers included NMR, IR, and such instead of X-ray crystallography.
Many had difficulties writing the balanced equation(s) for the formation of the nitronium ion.
Again, many had difficulty drawing the structural formula of the carbocation intermediate produced in the reaction.
Deducing the number of signals in the 1H NMR spectrum of nitrobenzene, which depend on the number of different hydrogen environments, was done poorly. Also, instead of relative areas, the common answer included chemical shift (ppm) values.
Benzoic acid, C6H5COOH, is another derivative of benzene.
Identify the wavenumber of one peak in the IR spectrum of benzoic acid, using section 26 of the data booklet.
Identify the spectroscopic technique that is used to measure the bond lengths in solid benzoic acid.
Outline one piece of physical evidence for the structure of the benzene ring.
Draw the structure of the conjugate base of benzoic acid showing all the atoms and all the bonds.
Outline why both C to O bonds in the conjugate base are the same length and suggest a value for them. Use section 10 of the data booklet.
The pH of an aqueous solution of benzoic acid at 298 K is 2.95. Determine the concentration of hydroxide ions in the solution, using section 2 of the data booklet.
Formulate the equation for the complete combustion of benzoic acid in oxygen using only integer coefficients.
The combustion reaction in (f)(ii) can also be classed as redox. Identify the atom that is oxidized and the atom that is reduced.
Suggest how benzoic acid, Mr = 122.13, forms an apparent dimer, Mr = 244.26, when dissolved in a non-polar solvent such as hexane.
State the reagent used to convert benzoic acid to phenylmethanol (benzyl alcohol), C6H5CH2OH.
Markscheme
Any wavenumber in the following ranges:
2500−3000 «cm−1» [✔]
1700−1750 «cm−1» [✔]
2850−3090 «cm−1» [✔]
X-ray «crystallography/spectroscopy» [✔]
Any one of:
«regular» hexagon
OR
all «H–C–C/C-C-C» angles equal/120º [✔]
all C–C bond lengths equal/intermediate between double and single
OR
bond order 1.5 [✔]
[✔]
Note: Accept Kekulé structures.
Negative sign must be shown in correct position.
electrons delocalized «across the O–C–O system»
OR
resonance occurs [✔]
122 «pm» < C–O < 143 «pm» [✔]
Note: Accept “delocalized π-bond”.
Accept “bond intermediate between single and double bond” or “bond order 1.5” for M1.
Accept any answer in range 123 to 142 pm.
ALTERNATIVE 1:
[H+] «= 10−2.95» = 1.122 × 10−3 «mol dm−3» [✔]
«[OH−] = =» 8.91 × 10−12 «mol dm−3» [✔]
ALTERNATIVE 2:
pOH = «14 − 2.95 =» 11.05 [✔]
«[OH−] = 10−11.05 =» 8.91 × 10−12 «mol dm−3» [✔]
Note: Award [2] for correct final answer.
Accept other methods.
2C6H5COOH (s) + 15O2 (g) → 14CO2 (g) + 6H2O (l)
correct products [✔]
correct balancing [✔]
Oxidized:
C/carbon «in C6H5COOH»
AND
Reduced:
O/oxygen «in O2» [✔]
«intermolecular» hydrogen bonding [✔]
Note: Accept diagram showing hydrogen bonding.
lithium aluminium hydride/LiAlH4 [✔]
Examiners report
Most candidates could identify a wavenumber or range of wavenumbers in the IR spectrum of benzoic acid.
Less than half the candidates identified x-ray crystallography as a technique used to measure bond lengths. There were many stating IR spectroscopy and quite a few random guesses.
Again less than half the candidates could accurately give a physical piece of evidence for the structure of benzene. Many missed the mark by not being specific, stating ‘all bonds in benzene with same length’ rather than ‘all C-C bonds in benzene have the same length’.
Very poorly answered with only 1 in 5 getting this question correct. Many did not show all the bonds and all the atoms or either forgot or misplaced the negative sign on the conjugate base.
This question was a challenge. Candidates were not able to explain the intermediate bond length and the majority suggested the value of either the bond length of C to O single bond or double bond.
Generally well done with a few calculating the pOH rather than the concentration of hydroxide ion asked for.
Most earned at least one mark by correctly stating the products of the reaction.
Another question where not reading correctly was a concern. Instead of identifying the atom that is oxidized and the atom that is reduced, answers included formulas of molecules or the atoms were reversed for the redox processes.
The other question where only 10 % of the candidates earned a mark. Few identified hydrogen bonding as the reason for carboxylic acids forming dimers. There were many G2 forms stating that the use of the word “dimer” is not in the syllabus, however the candidates were given that a dimer has double the molar mass and the majority seemed to understand that the two molecules joined together somehow but could not identify hydrogen bonding as the cause.
Very few candidates answered this part correctly and scored the mark. Common answers were H2SO4, HCl & Sn, H2O2. In general, strongest candidates gained the mark.
Dinitrogen monoxide, N2O, causes depletion of ozone in the stratosphere.
Different sources of N2O have different ratios of 14N : 15N.
The Lewis (electron dot) structure of the dinitrogen monoxide molecule can be represented as:
Outline why ozone in the stratosphere is important.
Dinitrogen monoxide in the stratosphere is converted to nitrogen monoxide, NO (g).
Write two equations to show how NO (g) catalyses the decomposition of ozone.
State one analytical technique that could be used to determine the ratio of 14N : 15N.
A sample of gas was enriched to contain 2 % by mass of 15N with the remainder being 14N.
Calculate the relative molecular mass of the resulting N2O.
Predict, giving two reasons, how the first ionization energy of 15N compares with that of 14N.
Explain why the first ionization energy of nitrogen is greater than both carbon and oxygen.
Nitrogen and carbon:
Nitrogen and oxygen:
State what the presence of alternative Lewis structures shows about the nature of the bonding in the molecule.
State, giving a reason, the shape of the dinitrogen monoxide molecule.
Deduce the hybridization of the central nitrogen atom in the molecule.
Markscheme
absorbs UV/ultraviolet light «of longer wavelength than absorbed by O2» [✔]
NO (g) + O3 (g) → NO2 (g) + O2 (g) [✔]
NO2 (g) + O3 (g) → NO (g) + 2O2 (g) [✔]
Note: Ignore radical signs.
Accept equilibrium arrows.
Award [1 max] for NO2 (g) + O (g) → NO (g) + O2 (g).
mass spectrometry/MS [✔]
« =» 14.02 [✔]
«Mr = (14.02 × 2) + 16.00 =» 44.04 [✔]
Any two:
same AND have same nuclear charge /number of protons/Zeff [✔]
same AND neutrons do not affect attraction/ionization energy/Zeff
OR
same AND neutrons have no charge [✔]
same AND same attraction for «outer» electrons [✔]
same AND have same electronic configuration/shielding [✔]
Note: Accept “almost the same”.
“Same” only needs to be stated once.
Nitrogen and carbon:
N has greater nuclear charge/«one» more proton «and electrons both lost from singly filled p-orbitals» [✔]
Nitrogen and oxygen:
O has a doubly filled «p-»orbital
OR
N has only singly occupied «p-»orbitals [✔]
Note: Accept “greater e– - e- repulsion in O” or “lower e– - e- repulsion in N”.
Accept box annotation of electrons for M2.
delocalization
OR
delocalized π-electrons [✔]
Note: Accept “resonance”.
linear AND 2 electron domains
OR
linear AND 2 regions of electron density [✔]
Note: Accept “two bonds AND no lone pairs” for reason.
sp [✔]
Examiners report
Candidates sometimes failed to identify how ozone works in chemical terms, referring to protects/deflects, i.e., the consequence rather than the mechanism.
Many candidates recalled the first equation for NO catalyzed decomposition of ozone only. Some considered other radical species.
All candidates, with very few exceptions, answered this correctly.
Most candidates were able to calculate the accurate mass of N2O, though quite a few candidates just calculated the mass of N and didn’t apply it to N2O, losing an accessible mark.
Many students realized that neutrons had no charge and could not affect IE significantly, but many others struggled a lot with this question since they considered that 15N would have a higher IE because they considered the greater mass of the nucleus would result in an increase of attraction of the electrons.
Mixed responses here; the explanation of higher IE for N with respect to C was less well explained, though it should have been the easiest. It was good to see that most candidates could explain the difference in IE of N and O, either mentioning paired/unpaired electrons or drawing box diagrams.
Most candidates identified resonance for this given Lewis representation.
Though quite a number of candidates suggested a linear shape correctly, they often failed to give a complete correct explanation, just mentioning the absence of lone pairs but not two bonds, instead of referring to electron domains.
Hybridisation of the N atom was correct in most cases.
Bromine can form the bromate(V) ion, BrO3−.
State the electron configuration of a bromine atom.
Sketch the orbital diagram of the valence shell of a bromine atom (ground state) on the energy axis provided. Use boxes to represent orbitals and arrows to represent electrons.
Draw two Lewis (electron dot) structures for BrO3−.
Determine the preferred Lewis structure based on the formal charge on the bromine atom, giving your reasons.
Predict, using the VSEPR theory, the geometry of the BrO3− ion and the O−Br−O bond angles.
Bromate(V) ions act as oxidizing agents in acidic conditions to form bromide ions.
Deduce the half-equation for this reduction reaction.
Bromate(V) ions oxidize iron(II) ions, Fe2+, to iron(III) ions, Fe3+.
Deduce the equation for this redox reaction.
Calculate the standard Gibbs free energy change, ΔGΘ, in J, of the redox reaction in (ii), using sections 1 and 24 of the data booklet.
EΘ (BrO3− / Br−) = +1.44 V
State and explain the magnetic property of iron(II) and iron(III) ions.
Markscheme
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
OR
[Ar] 4s2 3d10 4p5 ✔
Accept 3d before 4s.
Accept double-headed arrows.
Structure I - follows octet rule:
Structure II - does not follow octet rule:
Accept dots, crosses or lines to represent electron pairs.
«structure I» formal charge on Br = +2
OR
«structure II» formal charge on Br = 0/+1 ✔
structure II is preferred AND it produces formal charge closer to 0 ✔
Ignore any reference to formal charge on oxygen.
Geometry:
trigonal/pyramidal ✔
Reason:
three bonds AND one lone pair
OR
four electron domains ✔
O−Br−O angle:
107° ✔
Accept “charge centres” for “electron domains”.
Accept answers in the range 104–109°.
BrO3− (aq) + 6e− + 6H+ (aq) → Br− (aq) + 3H2O (l)
correct reactants and products ✔
balanced equation ✔
Accept reversible arrows.
BrO3− (aq) + 6Fe2+ (aq) + 6H+ (aq) → Br− (aq) + 3H2O (l) + 6Fe3+ (aq) ✔
EΘreaction = «+1.44 V – 0.77 V =» 0.67 «V» ✔
ΔGΘ = «–nFEΘreaction = – 6 × 96500 C mol–1 × 0.67 V =» –3.9 × 105 «J» ✔
both are paramagnetic ✔
«both» contain unpaired electrons ✔
Accept orbital diagrams for both ions showing unpaired electrons.
Examiners report
Hybridization of hydrocarbons affects their reactivity.
Experiments were carried out to investigate the mechanism of reaction between 2-chloropentane and aqueous sodium hydroxide.
Distinguish between a sigma and pi bond.
Identify the hybridization of carbon in ethane, ethene and ethyne.
State, giving a reason, if but-1-ene exhibits cis-trans isomerism.
State the type of reaction which occurs between but-1-ene and hydrogen iodide at room temperature.
Explain the mechanism of the reaction between but-1-ene with hydrogen iodide, using curly arrows to represent the movement of electron pairs.
State, giving a reason, if the product of this reaction exhibits stereoisomerism.
Deduce the rate expression for this reaction.
Deduce the units of the rate constant.
Determine the initial rate of reaction in experiment 4.
Deduce, with a reason, the mechanism of the reaction between 2-chloropentane and sodium hydroxide.
Discuss the reason benzene is more reactive with an electrophile than a nucleophile.
Markscheme
Sigma (σ) bond:
overlap «of atomic orbitals» along the axial / intermolecular axis / electron density is between nuclei
OR
head-on/end-to-end overlap «of atomic orbitals» ✔
Pi (π) bond:
overlap «of p-orbitals» above and below the internuclear axis/electron density above and below internuclear axis
OR
sideways overlap «of p-orbitals» ✔
Accept a suitable diagram.
All 3 required for mark.
no AND 2 groups on a carbon «in the double bond» are the same/hydrogen «atoms»
OR
no AND molecule produced by rearranging atoms bonded on a carbon «in the double bond» is the same as the original ✔
«electrophilic» addition ✔
Do not allow nucleophilic addition.
curly arrow going from C=C to H of HI AND curly arrow showing I leaving ✔
representation of carbocation ✔
curly arrow going from lone pair/negative charge on I– to C+ ✔
2-iodobutane formed ✔
Penalize incorrect bond, e.g. –CH–H3C or –CH3C once only.
yes AND has a carbon attached to four different groups
OR
yes AND it contains a chiral carbon ✔
Accept yes AND mirror image of molecule different to original/non-superimposable on original.
«rate =» k[NaOH][C5H11Cl] ✔
mol–1 dm3 s–1 ✔
ALTERNATIVE 1:
«k = » 1.25 «mol–1 dm3 s–1» ✔
«rate = 1.25 mol–1 dm3 s–1 × 0.60 mol dm–3 × 0.25 mol dm–3»
1.9 x 10–1 «mol dm–3 s–1» ✔
ALTERNATIVE 2:
«[NaOH] exp. 4 is 3 × exp. 1»
«[C5H11Cl] exp. 4 is 2.5 × exp. 1»
«exp. 4 will be » 7.5× faster ✔
1.9 x 10–1 «mol dm–3 s–1» ✔
Award [2] for correct final answer.
SN2 AND rate depends on both OH– and 2-chloropentane ✔
Accept E2 AND rate depends on both OH– and 2-chloropentane.
delocalized electrons/pi bonds «around the ring»
OR
molecule has a region of high electron density/negative charge ✔
electrophiles are attracted/positively charged AND nucleophiles repelled/negatively charged ✔
Do not accept just “nucleophiles less attracted” for M2.
Accept “benzene AND nucleophiles are both electron rich” for “repels nucleophiles”.
Examiners report
Propane and propene are members of different homologous series.
(i) Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms.
(ii) State the number of sigma (σ) and pi (π) bonds in propane and propene.
Construct the mechanism of the formation of 2-bromopropane from hydrogen bromide and propene using curly arrows to denote the movement of electrons.
Markscheme
i
ii
Award [1] for two or three correct answers.
Award [2] for all four correct.
curly arrow going from C=C to H of HBr and curly arrow showing Br leaving
representation of carbocation
curly arrow going from lone pair/negative charge on Br– to C+
Award [2 max] for formation of 1-bromopropane.
Examiners report
Calcium carbide, CaC2, is an ionic solid.
Describe the nature of ionic bonding.
Describe how the relative atomic mass of a sample of calcium could be determined from its mass spectrum.
When calcium compounds are introduced into a gas flame a red colour is seen; sodium compounds give a yellow flame. Outline the source of the colours and why they are different.
Suggest two reasons why solid calcium has a greater density than solid potassium.
Outline why solid calcium is a good conductor of electricity.
Sketch a graph of the first six ionization energies of calcium.
Calcium carbide reacts with water to form ethyne and calcium hydroxide.
CaC2(s) + H2O(l) → C2H2(g) + Ca(OH)2(aq)
Estimate the pH of the resultant solution.
Describe how sigma (σ) and pi () bonds are formed.
Deduce the number of σ and bonds in a molecule of ethyne.
Markscheme
electrostatic attraction AND oppositely charged ions
[1 mark]
multiply relative intensity by «m/z» value of isotope
OR
find the frequency of each isotope
sum of the values of products/multiplication «from each isotope»
OR
find/calculate the weighted average
Award [1 max] for stating “m/z values of isotopes AND relative abundance/intensity” but not stating these need to be multiplied.
[2 marks]
«promoted» electrons fall back to lower energy level
energy difference between levels is different
Accept “Na and Ca have different nuclear charge” for M2.
[2 marks]
Any two of:
stronger metallic bonding
smaller ionic/atomic radius
two electrons per atom are delocalized
OR
greater ionic charge
greater atomic mass
Do not accept just “heavier” or “more massive” without reference to atomic mass.
[2 marks]
delocalized/mobile electrons «free to move»
[1 mark]

general increase
only one discontinuity between “IE2” and “IE3”
[2 marks]
pH > 7
Accept any specific pH value or range of values above 7 and below 14.
[1 mark]
sigma (σ):
overlap «of atomic orbitals» along the axial/internuclear axis
OR
head-on/end-to-end overlap «of atomic orbitals»
pi ():
overlap «of p-orbitals» above and below the internuclear axis
OR
sideways overlap «of p-orbitals»
Award marks for suitable diagrams.
[2 marks]
sigma (σ): 3
AND
pi (): 2
[1 mark]
Examiners report
Organomagnesium compounds can react with carbonyl compounds. One overall equation is:
Compound B can also be prepared by reacting an alkene with water.
Iodomethane is used to prepare CH3Mg. It can also be converted into methanol:
CH3 + HO– → CH3OH + –
State the name of Compound B, applying International Union of Pure and Applied Chemistry (IUPAC) rules.
Compound A and Compound B are both liquids at room temperature and pressure. Identify the strongest intermolecular force between molecules of Compound A.
State the number of (sigma) and (pi) bonds in Compound A.
Deduce the hybridization of the central carbon atom in Compound A.
Identify the isomer of Compound B that exists as optical isomers (enantiomers).
Draw the structural formula of the alkene required.
Explain why the reaction produces more (CH3)3COH than (CH3)2CHCH2OH.
Deduce the structural formula of the repeating unit of the polymer formed from this alkene.
Deduce what would be observed when Compound B is warmed with acidified aqueous potassium dichromate (VI).
Identify the type of reaction.
Outline the requirements for a collision between reactants to yield products.
Explain the mechanism of the reaction using curly arrows to represent the movement of electron pairs.
The polarity of the carbon–halogen bond, C–X, facilitates attack by HO–.
Outline, giving a reason, how the bond polarity changes going down group 17.
Markscheme
2-methylpropan-2-ol /2-methyl-2-propanol ✔
Accept methylpropan-2-ol/ methyl-2-propanol.
Do not accept 2-methylpropanol.
dipole-dipole ✔
Do not accept van der Waals’ forces.
: 9
AND
: 1 ✔
sp2 ✔
butan-2-ol/CH3CH(OH)C2H5 ✔
carbocation formed from (CH3)3COH is more stable / (CH3)3C+ is more stable than (CH3)2CHCH2+ ✔
«because carbocation has» greater number of alkyl groups/lower charge on the atom/higher e- density
OR
«greater number of alkyl groups» are more electron releasing
OR
«greater number of alkyl groups creates» greater inductive/+I effect ✔
Do not award any marks for simply quoting Markovnikov’s rule.
Do not penalize missing brackets or n.
Do not award mark if continuation bonds are not shown.
no change «in colour/appearance/solution» ✔
«nucleophilic» substitution
OR
SN2 ✔
Accept “hydrolysis”.
Accept SN1
energy/E ≥ activation energy/Ea ✔
correct orientation «of reacting particles»
OR
correct geometry «of reacting particles» ✔
curly arrow going from lone pair/negative charge on O in -OH to C ✔
curly arrow showing I leaving ✔
representation of transition state showing negative charge, square brackets and partial bonds ✔
Accept OH- with or without the lone pair.
Do not allow curly arrows originating on H, rather than the -, in OH-.
Accept curly arrows in the transition state.
Do not penalize if HO and I are not at 180°.
Do not award M3 if OH–C bond is represented.
Award [2 max] if SN1 mechanism shown.
decreases/less polar AND electronegativity «of the halogen» decreases ✔
Accept “decreases” AND a correct comparison of the electronegativity of two halogens.
Accept “decreases” AND “attraction for valence electrons decreases”.
Examiners report
Naming the organic compound using IUPAC rules was generally done well.
Mediocre performance in stating the number of σ (sigma) and π (pi) bonds in propanone; the common answer was 3 σ and 1 π instead of 9 σ and 1 π, suggesting the three C-H σ bonds in each of the two methyl groups were ignored.
sp2 hybridization of the central carbon atom in the ketone was very done well.
Mediocre performance; some identified 2-methylpropan-1-ol or -2-ol, instead butan-2-ol/CH3CH(OH)C2H5 as the isomer that exists as an optical isomer.
Good performance; some had a H and CH3 group on each C atom across double bond instead of having two H atoms on one C and two CH3 groups on the other.
Poor performance, particularly in light of past feedback provided in similar questions since there was repeated reference simply to Markovnikov's rule, without any explanation.
Mediocre performance; deducing structural formula of repeating unit of the polymer was challenging in which continuation bonds were sometimes missing, or structure included a double bond or one of the CH3 group was missing.
Mediocre performance; deducing whether the tertiary alcohol could be oxidized solicited mixed responses ranging from the correct one, namely no change (in colour, appearance or solution), to tertiary alcohol will be reduced, or oxidized, or colour will change will occur, and such.
Excellent performance on the type of reaction but with some incorrect answers such as alkane substitution, free radical substitution or electrophilic substitution.
Good performance. For the requirements for a collision between reactants to yield products, some suggested necessary, sufficient or enough energy or even enough activation energy instead of energy/E ≥ activation energy/Ea.
Mechanism for SN2 not done well. Often the negative charge on OH was missing, the curly arrow was not going from lone pair/negative charge on O in -OH to C, or the curly arrow showing I leaving placed incorrectly and specially the negative charge was missing in the transition state. Formation of a carbocation intermediate indicating SN1 mechanism could score a maximum of 2 marks.
Good performance on how the polarity of C-X bond changes going down group 17.
A compound with a molecular formula C7H14O produced the following high resolution 1H NMR spectrum.
Deduce what information can be obtained from the 1H NMR spectrum.
Identify the functional group that shows stretching at 1710 cm–1 in the infrared spectrum of this compound using section 26 of the data booklet and the 1H NMR.
Suggest the structural formula of this compound.
Bromine was added to hexane, hex-1-ene and benzene. Identify the compound(s) which will react with bromine in a well-lit laboratory.
Deduce the structural formula of the main organic product when hex-1-ene reacts with hydrogen bromide.
State the reagents and the name of the mechanism for the nitration of benzene.
Outline, in terms of the bonding present, why the reaction conditions of halogenation are different for alkanes and benzene.
Below are two isomers, A and B, with the molecular formula C4H9Br.
Explain the mechanism of the nucleophilic substitution reaction with NaOH(aq) for the isomer that reacts almost exclusively by an SN2 mechanism using curly arrows to represent the movement of electron pairs.
Markscheme
Number of hydrogen environments: 3
Ratio of hydrogen environments: 2:3:9
Splitting patterns: «all» singlets
Accept any equivalent ratios such as 9:3:2.
Accept “no splitting”.
[3 marks]
carbonyl
OR
C=O
Accept “ketone” but not “aldehyde”.
[1 mark]
Accept (CH3)3CCH2COCH3.
Award [1] for any aldehyde or ketone with C7H14O structural formula.
[2 marks]
hexane AND hex-1-ene
Accept “benzene AND hexane AND hex-1-ene”.
[1 mark]
CH3CH2CH2CH2CHBrCH3
Accept displayed formula but not molecular formula.
[1 mark]
Reagents: «concentrated» sulfuric acid AND «concentrated» nitric acid
Name of mechanism: electrophilic substitution
[2 marks]
benzene has «delocalized» bonds «that are susceptible to electrophile attack» AND alkanes do not
Do not accept “benzene has single and double bonds”.
[1 mark]
curly arrow going from lone pair/negative charge on O in –OH to C
curly arrow showing Br leaving
representation of transition state showing negative charge, square brackets and partial bonds
Accept OH– with or without the lone pair.
Do not allow curly arrows originating on H in OH–.
Accept curly arrows in the transition state.
Do not penalize if HO and Br are not at 180°.
Do not award M3 if OH–C bond is represented.
Award [2 max] if wrong isomer is used.
[3 marks]
Examiners report
The equations show steps in the formation and decomposition of ozone in the stratosphere, some of which absorb ultraviolet light.
Step 1 O2 → 2O•
Step 2 O• + O2 → O3
Step 3 O3 → O• + O2
Step 4 O• + O3 → 2O2
Draw the Lewis structures of oxygen, O2, and ozone, O3.
Outline why both bonds in the ozone molecule are the same length and predict the bond length in the ozone molecule. Refer to section 10 of the data booklet.
Reason:
Length:
Predict the bond angle in the ozone molecule.
Discuss how the different bond strengths between the oxygen atoms in O2 and O3 in the ozone layer affect radiation reaching the Earth’s surface.
Identify the steps which absorb ultraviolet light.
Determine, showing your working, the wavelength, in m, of ultraviolet light absorbed by a single molecule in one of these steps. Use sections 1, 2 and 11 of the data booklet.
Ozone depletion is catalysed by nitrogen monoxide, NO, which is produced in aircraft and motor vehicle engines, and has the following Lewis structure.
Show how nitrogen monoxide catalyses the decomposition of ozone, including equations in your answer.
Markscheme
NOTES: Coordinate bond may be represented by an arrow.
Do not accept delocalized structure for ozone.
resonance «structures»
OR
delocalization of «the double/pi bond» electrons ✔
121 «pm» < length < 148 «pm» ✔
NOTE: Accept any length between these two values.
any value from 110°–119° ✔
«bond» in O2 stronger than in O3 ✔
ozone absorbs lower frequency/energy «radiation than oxygen»
OR
ozone absorbs longer wavelength «radiation than oxygen» ✔
NOTE: Accept ozone «layer» absorbs a range of frequencies.
steps 1 AND 3 ✔
ALTERNATIVE 1:
for oxygen:
✔
✔
ALTERNATIVE 2:
for ozone:
similar calculation using 200 < bond enthalpy < 400 for ozone, such as
✔
✔
NOTE: Award [2] for correct final answer.
•NO + O3 → •NO2 + O2 ✔
•NO2 + O3 → •NO + 2O2 ✔
NOTE: Accept •NO2 → •NO + •O AND •O + O3 → 2O2 for M2.
Examiners report
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) (H2N)2CO(g) + H2O(g) ΔH < 0
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.

Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.

Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
State the equilibrium constant expression, Kc.
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
Calculate the maximum volume of CO2, in cm3, produced at STP by the combustion of 0.600 g of urea, using sections 2 and 6 of the data booklet.
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
The mass spectrum of urea is shown below.

Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.

Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Predict the splitting pattern of the 1H NMR spectrum of urea.
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
Markscheme
molar mass of urea «4 1.01 + 2 14.01 + 12.01 + 16.00» = 60.07 «g mol-1»
«% nitrogen = 100 =» 46.65 «%»
Award [2] for correct final answer.
Award [1 max] for final answer not to two decimal places.
[2 marks]
«cost» increases AND lower N% «means higher cost of transportation per unit of nitrogen»
OR
«cost» increases AND inefficient/too much/about half mass not nitrogen
Accept other reasonable explanations.
Do not accept answers referring to safety/explosions.
[1 mark]

Note: Urea’s structure is more complex than that predicted from VSEPR theory.
[3 marks]
n(KNCO) «= 0.0500 dm3 0.100 mol dm–3» = 5.00 10–3 «mol»
«mass of urea = 5.00 10–3 mol 60.07 g mol–1» = 0.300 «g»
Award [2] for correct final answer.
[2 marks]
[1 mark]
«Kc» decreases AND reaction is exothermic
OR
«Kc» decreases AND ΔH is negative
OR
«Kc» decreases AND reverse/endothermic reaction is favoured
[1 mark]
ln K « = » = –20
«Kc =» 2 10–9
OR
1.69 10–9
OR
10–9
Accept range of 20-20.2 for M1.
Award [2] for correct final answer.
[2 marks]
Any one of:
urea has greater molar mass
urea has greater electron density/greater London/dispersion
urea has more hydrogen bonding
urea is more polar/has greater dipole moment
Accept “urea has larger size/greater van der Waals forces”.
Do not accept “urea has greater intermolecular forces/IMF”.
[1 mark]

Award [1] for each correct interaction.
If lone pairs are shown on N or O, then the lone pair on N or one of the lone pairs on O MUST be involved in the H-bond.
Penalize solid line to represent H-bonding only once.
[2 marks]
2(H2N)2CO(s) + 3O2(g) → 4H2O(l) + 2CO2(g) + 2N2(g)
correct coefficients on LHS
correct coefficients on RHS
Accept (H2N)2CO(s) + O2(g) → 2H2O(l) + CO2(g) + N2(g).
Accept any correct ratio.
[2 marks]
«V = 22700 cm3 mol–1 =» 227 «cm3»
[1 mark]
lone/non-bonding electron pairs «on nitrogen/oxygen/ligand» given to/shared with metal ion
co-ordinate/dative/covalent bonds
[2 marks]
lone pairs on nitrogen atoms can be donated to/shared with C–N bond
OR
C–N bond partial double bond character
OR
delocalization «of electrons occurs across molecule»
OR
slight positive charge on C due to C=O polarity reduces C–N bond length
[1 mark]
60: CON2H4+
44: CONH2+
Accept “molecular ion”.
[2 marks]
3450 cm–1: N–H
1700 cm–1: C=O
Do not accept “O–H” for 3450 cm–1.
[2 marks]
1
[2 marks]
singlet
Accept “no splitting”.
[1 mark]
acts as internal standard
OR
acts as reference point
one strong signal
OR
12 H atoms in same environment
OR
signal is well away from other absorptions
Accept “inert” or “readily removed” or “non-toxic” for M1.
[2 marks]
Examiners report
PCl5(g) and Cl2(g) were placed in a sealed flask and allowed to reach equilibrium at 200 °C. The enthalpy change, ΔH, for the decomposition of PCl5(g) is positive.
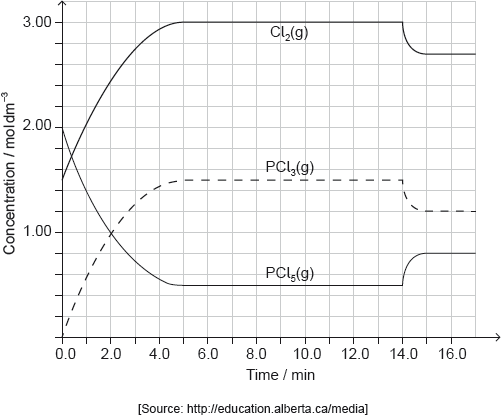
Deduce the Lewis (electron dot) structure and molecular geometry and the bond angles of PCl3.
Markscheme
Lewis structure:
Molecular geometry:
trigonal/triangular pyramidal
Bond angles:
< 109.5°
Penalize missing lone pairs once only between this question and 4(b)(ii).
Accept any combination of lines, dots or crosses to represent electrons.
Do not apply ECF.
Do not accept answer equal to or less than 90°.
Literature value is 100.1°.
[3 marks]
Examiners report
Chlorine undergoes many reactions.
of manganese(IV) oxide was added to of .
Chlorine gas reacts with water to produce hypochlorous acid and hydrochloric acid.
is a common chlorofluorocarbon, .
State the full electron configuration of the chlorine atom.
State, giving a reason, whether the chlorine atom or the chloride ion has a larger radius.
Outline why the chlorine atom has a smaller atomic radius than the sulfur atom.
The mass spectrum of chlorine is shown.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Outline the reason for the two peaks at and .
Explain the presence and relative abundance of the peak at .
Calculate the amount, in , of manganese(IV) oxide added.
Determine the limiting reactant, showing your calculations.
Determine the excess amount, in , of the other reactant.
Calculate the volume of chlorine, in , produced if the reaction is conducted at standard temperature and pressure (STP). Use section 2 of the data booklet.
State the oxidation state of manganese in and .
Deduce, referring to oxidation states, whether is an oxidizing or reducing agent.
Hypochlorous acid is considered a weak acid. Outline what is meant by the term weak acid.
State the formula of the conjugate base of hypochlorous acid.
Calculate the concentration of in a solution with a .
State the type of reaction occurring when ethane reacts with chlorine to produce chloroethane.
Predict, giving a reason, whether ethane or chloroethane is more reactive.
Explain the mechanism of the reaction between chloroethane and aqueous sodium hydroxide, , using curly arrows to represent the movement of electron pairs.
Ethoxyethane (diethyl ether) can be used as a solvent for this conversion.
Draw the structural formula of ethoxyethane
Deduce the number of signals and chemical shifts with splitting patterns in the 1H NMR spectrum of ethoxyethane. Use section 27 of the data booklet.
Calculate the percentage by mass of chlorine in .
Comment on how international cooperation has contributed to the lowering of emissions responsible for ozone depletion.
s produce chlorine radicals. Write two successive propagation steps to show how chlorine radicals catalyse the depletion of ozone.
Markscheme
✔
Do not accept condensed electron configuration.
AND more «electron–electron» repulsion ✔
Accept AND has an extra electron.
has a greater nuclear charge/number of protons/ «causing a stronger pull on the outer electrons» ✔
same number of shells
OR
same «outer» energy level
OR
similar shielding ✔
«two major» isotopes «of atomic mass and » ✔
«diatomic» molecule composed of «two» chlorine-37 atoms ✔
chlorine-37 is the least abundant «isotope»
OR
low probability of two «isotopes» occurring in a molecule ✔
✔
✔
AND is the limiting reactant ✔
Accept other valid methods of determining the limiting reactant in M2.
✔
✔
Accept methods employing .
✔
✔
oxidizing agent AND oxidation state of changes from to /decreases ✔
partially dissociates/ionizes «in water» ✔
✔
✔
«free radical» substitution/ ✔
Do not accept electrophilic or nucleophilic substitution.
chloroethane AND C–Cl bond is weaker/ than C–H bond/
OR
chloroethane AND contains a polar bond ✔
Accept “chloroethane AND polar”.
curly arrow going from lone pair/negative charge on in −OH to ✔
curly arrow showing leaving ✔
representation of transition state showing negative charge, square brackets and partial bonds ✔
Accept with or without the lone pair.
Do not accept curly arrows originating on in .
Accept curly arrows in the transition state.
Do not penalize if and are not at 180°.
Do not award M3 if bond is represented.
/ ✔
Accept .
2 «signals» ✔
0.9−1.0 AND triplet ✔
3.3−3.7 AND quartet ✔
Accept any values in the ranges.
Award [1] for two correct chemical shifts or two correct splitting patterns.
✔
✔
Award [2] for correct final answer.
Any of:
research «collaboration» for alternative technologies «to replace s»
OR
technologies «developed»/data could be shared
OR
political pressure/Montreal Protocol/governments passing legislations ✔
Do not accept just “collaboration”.
Do not accept any reference to as greenhouse gas or product of fossil fuel combustion.
Accept reference to specific measures, such as agreement on banning use/manufacture of s.
✔
OR
✔
Penalize missing/incorrect radical dot (∙) once only.
Examiners report
Well answered question with 90% of candidates correctly identifying the complete electron configuration for chlorine.
Most candidates could correctly explain the relative sizes of chlorine atom and chloride ion.
Fairly well answered though some candidates missed M2 for not recognizing the same number of shells affected.
More than 80% could identify that the two peaks in the MS of chlorine are due to different isotopes.
Not well answered. Some candidates were able to identify m/z 74 being due to the m/z of two Cl-37 atoms, however fewer candidates were able to explain the relative abundance of the isotope.
Stoichiometric calculations were generally well done and over 90% could calculate mol from a given mass.
90% of candidates earned full marks on this 2-mark question involving finding a limiting reactant.
Surprisingly, quite a number of candidates struggled with the quantity of excess reactant despite correctly identifying limiting reactant previously.
Most candidates could find the volume of gas produced in a reaction under standard conditions.
More than 90% could identify the oxidation number of manganese in both MnO2 and MnCl2.
Most candidates stated that MnO2 is an oxidizing agent in the reaction but many did not get the mark because there was no reference to oxidation states.
Another well answered 1-mark question where candidates correctly identified a weak acid as an acid which partially dissociates in water.
Roughly ⅓ of the candidates failed to identify the conjugate base, perhaps distracted by the fact it was not contained in the equation given.
Vast majority of candidates could calculate the concentration of H+ (aq) in a HClO (aq) solution with a pH =3.61.
Many identified the reaction of chlorine with ethane as free-radical substitution, or just substitution, with some erroneously stating nucleophilic or electrophilic substitution.
The underlying reasons for the relative reactivity of ethane and chloroethane were not very well known with a few giving erroneous reasons and some stating ethane more reactive.
Few earned full marks for the curly arrow mechanism of the reaction between sodium hydroxide and chloroethane. Mistakes being careless curly arrow drawing, inappropriate –OH notation, curly arrows from the hydrogen or from the carbon to the C–Cl bond, or a method that missed the transition state.
Approximately 60% could draw ethoxyethane however many demonstrated little knowledge of structure of an ether molecule.
A poorly answered question with some getting full marks on this 1HNMR spectrum of ethoxyethane question. Very few could identify all 3 of number of signals, chemical shift, and splitting pattern.
Another good example of candidates being well rehearsed in calculations with 90% earning 2/2 on this question of calculation percentage by mass composition.
Somewhat disappointing answers on this question about how international cooperation has contributed to the lowering of CFC emissions. Many gave vague answers and some referred to carbon emissions and global warming.
Few could construct the propagation equations showing how CFCs affect ozone, and many lost marks by failing to identify ClO· as a radical.
Copper forms two chlorides, copper(I) chloride and copper(II) chloride.
Two electrolysis cells were assembled using graphite electrodes and connected in series as shown.
Copper(I) chloride undergoes a disproportionation reaction, producing copper(II) chloride and copper.
2Cu+ (aq) → Cu (s) + Cu2+ (aq)
Dilute copper(II) chloride solution is light blue, while copper(I) chloride solution is colourless.
State the electron configuration of the Cu+ ion.
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, ΔHθ, in kJ, for this reaction, using section 12 of the data booklet.
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label Ea (cat).
Explain how the catalyst increases the rate of the reaction.
Solid copper(II) chloride absorbs moisture from the atmosphere to form a hydrate of formula CuCl2•H2O.
A student heated a sample of hydrated copper(II) chloride, in order to determine the value of . The following results were obtained:
Mass of crucible = 16.221 g
Initial mass of crucible and hydrated copper(II) chloride = 18.360 g
Final mass of crucible and anhydrous copper(II) chloride = 17.917 g
Determine the value of .
State how current is conducted through the wires and through the electrolyte.
Wires:
Electrolyte:
Write the half-equation for the formation of gas bubbles at electrode 1.
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
Deduce the half-equation for the formation of the gas identified in (c)(iii).
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
Calculate the cell potential at 298 K for the disproportionation reaction, in V, using section 24 of the data booklet.
Comment on the spontaneity of the disproportionation reaction at 298 K.
Calculate the standard Gibbs free energy change, ΔGθ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
Deduce, giving a reason, the sign of the standard enthalpy change, ΔHθ, for the disproportionation reaction at 298 K.
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
Describe how the blue colour is produced in the Cu(II) solution. Refer to section 17 of the data booklet.
Deduce why the Cu(I) solution is colourless.
When excess ammonia is added to copper(II) chloride solution, the dark blue complex ion, [Cu(NH3)4(H2O)2]2+, forms.
State the molecular geometry of this complex ion, and the bond angles within it.
Molecular geometry:
Bond angles:
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
Markscheme
[Ar] 3d10
OR
1s2 2s2 2p6 3s2 3p6 3d10 ✔
ΔHθ = ΣΔHθf (products) − ΣΔHθf (reactants) ✔
ΔHθ = 2(−241.8 «kJ mol−1») − 4(−92.3 «kJ mol−1») = −114.4 «kJ» ✔
NOTE: Award [2] for correct final answer.
Ea (cat) to the left of Ea ✔
peak lower AND Ea (cat) smaller ✔
«catalyst provides an» alternative pathway ✔
«with» lower Ea
OR
higher proportion of/more particles with «kinetic» E ≥ Ea(cat) «than Ea» ✔
mass of H2O = «18.360 g – 17.917 g =» 0.443 «g» AND mass of CuCl2 = «17.917 g – 16.221 g =» 1.696 «g» ✔
moles of H2O = «=» 0.0246 «mol»
OR
moles of CuCl2 =«= » 0.0126 «mol» ✔
«water : copper(II) chloride = 1.95 : 1»
« =» 2 ✔
NOTE: Accept « =» 1.95.
NOTE: Award [3] for correct final answer.
Wires:
«delocalized» electrons «flow» ✔
Electrolyte:
«mobile» ions «flow» ✔
2Cl− → Cl2 (g) + 2e−
OR
Cl− → Cl2 (g) + e− ✔
NOTE: Accept e for e−.
«electrode» 3 AND oxygen/O2 ✔
NOTE: Accept chlorine/Cl2.
2H2O (l) → 4H+ (aq) + O2 (g) + 4e– ✔
NOTE: Accept 2Cl– (aq) → Cl2 (g) + 2e–.
Accept 4OH− → 2H2O + O2 + 4e−
enthalpy of solution = lattice enthalpy + enthalpies of hydration «of Cu2+ and Cl−» ✔
«+2824 kJ mol–1 − 2161 kJ mol–1 − 2(359 kJ mol–1) =» −55 «kJ mol–1» ✔
NOTE: Accept enthalpy cycle.
Award [2] for correct final answer.
Eθ = «+0.52 – 0.15 = +» 0.37 «V» ✔
spontaneous AND Eθ positive ✔
ΔGθ = «−nFE = −1 mol × 96 500 C Mol–1 × 0.37 V=» −36 000 J/−36 kJ ✔
NOTE: Accept “−18 kJ mol–1 «per mole of Cu+»”.
Do not accept values of n other than 1.
Apply SF in this question.
Accept J/kJ or J mol−1/kJ mol−1 for units.
2 mol (aq) → 1 mol (aq) AND decreases ✔
NOTE: Accept “solid formed from aqueous solution AND decreases”.
Do not accept 2 mol → 1 mol without (aq).
ΔGθ < 0 AND ΔSθ < 0 AND ΔHθ < 0
OR
ΔGθ + TΔSθ < 0 AND ΔHθ < 0 ✔
TΔS more negative «reducing spontaneity» AND stability increases ✔
NOTE: Accept calculation showing non-spontaneity at 433 K.
«ligands cause» d-orbitals «to» split ✔
light absorbed as electrons transit to higher energy level «in d–d transitions»
OR
light absorbed as electrons promoted ✔
energy gap corresponds to «orange» light in visible region of spectrum ✔
colour observed is complementary ✔
full «3»d sub-level/orbitals
OR
no d–d transition possible «and therefore no colour» ✔
octahedral AND 90° «180° for axial» ✔
NOTE: Accept square-based bi-pyramid.
Any two of:
ligand/chloride ion Lewis base AND donates e-pair ✔
not Brønsted–Lowry base AND does not accept proton/H+ ✔
Lewis definition extends/broader than Brønsted–Lowry definition ✔
Examiners report
Both vinegar (a dilute aqueous solution of ethanoic acid) and bleach are used as cleaning agents.
Bleach reacts with ammonia, also used as a cleaning agent, to produce the poisonous compound chloramine, NH2Cl.
Outline why ethanoic acid is classified as a weak acid.
A solution of bleach can be made by reacting chlorine gas with a sodium hydroxide solution.
Cl2 (g) + 2NaOH (aq) ⇌ NaOCl (aq) + NaCl (aq) + H2O (l)
Suggest, with reference to Le Châtelier’s principle, why it is dangerous to mix vinegar and bleach together as cleaners.
Draw a Lewis (electron dot) structure of chloramine.
State the hybridization of the nitrogen atom in chloramine.
Deduce the molecular geometry of chloramine and estimate its H–N–H bond angle.
Molecular geometry:
H–N–H bond angle:
State the type of bond formed when chloramine is protonated.
Sketch a graph of pH against volume of hydrochloric acid added to ammonia solution, showing how you would determine the pKa of the ammonium ion.
Suggest a suitable indicator for the titration, using section 22 of the data booklet.
Explain, using two equations, how an equimolar solution of ammonia and ammonium ions acts as a buffer solution when small amounts of acid or base are added.
Markscheme
partial dissociation «in aqueous solution» [✔]
ethanoic acid/vinegar reacts with NaOH [✔]
moves equilibrium to left/reactant side [✔]
releases Cl2 (g)/chlorine gas
OR
Cl2 (g)/chlorine gas is toxic [✔]
Note: Accept “ethanoic acid produces H+ ions”
Accept “ethanoic acid/vinegar reacts with NaOCl”.
Do not accept “2CH3COOH + NaOCl + NaCl → 2CH3COONa + Cl2 + H2O” as it does not refer to equilibrium.
Accept suitable molecular or ionic equations for M1 and M3.
[✔]
Note: Accept any combination of dots/crosses or lines to represent electron pairs.
sp3 [✔]
Molecular geometry:
«trigonal» pyramidal [✔]
H–N–H bond angle:
107° [✔]
Note: Accept angles in the range of 100–109.
covalent/dative/coordinate [✔]
correct shape of graph AND vertical drop at Vn [✔]
pKa = pH at /half neutralization/half equivalence [✔]
Note: M1: must show buffer region at pH > 7 and equivalence point at pH < 7. Graph must start below pH = 14.
methyl orange
OR
bromophenol blue
OR
bromocresol green
OR
methyl red [✔]
NH3 (aq) + H+ (aq) → NH4 + (aq) [✔]
NH4 + (aq) + OH− (aq) → NH3 (aq) + H2O(l) [✔]
Note: Accept reaction arrows or equilibrium signs in both equations.
Award [1 max], based on two correct reverse equations but not clearly showing reacting with acid or base but rather dissociation.
Examiners report
Majority of candidates understood weak acids do not fully dissociate.
The average score was 1 out 3. Many could not suggest why it is dangerous to mix chlorine with vinegar. Most students gained at least one mark for stating that “chlorine gas will be produced” but couldn’t link it to equilibrium ideas.
Most candidates correctly drew the Lewis structure of chloramine. Some left off lone pair electrons.
Mostly correct with a surprising number stating sp or sp2 hybridization.
Generally well done with some candidates misinterpreting the bond angle from the stated geometry.
“Ionic bond”, “hydrogen bond” and “intermolecular forces” were some common answers.
Quite poorly done with many candidates not indicating a vertical drop but rather a weak acid/weak base curve. Some did not have the correct location for the equivalence point.
Generally well done although a number of candidates chose bromothymol blue as a suitable indicator for weak base with a strong acid.
Nearly 30 % of candidates did not attempt to answer this question about buffer equations. It was also poorly answered because equations were not used to explain buffer action or the dissociation equations for the base and acid were given rather than their reactions with H+ or OH- .
White phosphorus is an allotrope of phosphorus and exists as P4.
An equilibrium exists between PCl3 and PCl5.
PCl3 (g) + Cl2 (g) PCl5 (g)
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
Write an equation for the reaction of white phosphorus (P4) with chlorine gas to form phosphorus trichloride (PCl3).
Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl bond angle in PCl3.
Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
Calculate the entropy change, ΔS, in J K−1 mol−1, for this reaction.
Chemistry 2e, Chpt. 21 Nuclear Chemistry, Appendix G: Standard Thermodynamic Properties for Selected Substances https://openstax.org/books/chemistry-2e/pages/g-standard-thermodynamic-properties-for- selectedsubstances# page_667adccf-f900-4d86-a13d-409c014086ea © 1999-2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License. (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/.
Calculate the Gibbs free energy change (ΔG), in kJ mol−1, for this reaction at 25 °C. Use section 1 of the data booklet.
If you did not obtain an answer in c(i) or c(ii) use −87.6 kJ mol−1 and −150.5 J mol−1 K−1 respectively, but these are not the correct answers.
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
State the equilibrium constant expression, Kc, for this reaction.
State, with a reason, the effect of an increase in temperature on the position of this equilibrium.
Markscheme
Accept any diagram with each P joined to the other three.
Accept any combination of dots, crosses and lines.
P4 (s) + 6Cl2 (g) → 4PCl3 (l) ✔
Electron domain geometry: tetrahedral ✔
Molecular geometry: trigonal pyramidal ✔
Bond angle: 100«°» ✔
Accept any value or range within the range 91−108«°» for M3.
PCl5 is non-polar:
symmetrical
OR
dipoles cancel ✔
PCl4F is polar:
P–Cl has a different bond polarity than P–F ✔
non-symmetrical «dipoles»
OR
dipoles do not cancel ✔
Accept F more electronegative than/different electronegativity to Cl for M2.
«−398.9 kJ mol−1 − (−306.4 kJ mol−1) =» −92.5 «kJ mol−1» ✔
«ΔS = 364.5 J K–1 mol–1 – (311.7 J K–1 mol–1 + 223.0 J K–1 mol–1)=» –170.2 «J K–1 mol–1» ✔
«ΔS =» –0.1702 «kJ mol–1 K–1»
OR
298 «K» ✔
«ΔG = –92.5 kJ mol–1 – (298 K × –0.1702 kJ mol–1 K–1) =» –41.8 «kJ mol–1» ✔
Award [2] for correct final answer.
If –87.6 and -150.5 are used then –42.8.
«ΔG = –41.8 kJ mol–1 = × 298 K × lnK»
OR
«ΔG = –41800 J mol–1 = –8.31 J mol–1 K–1 × 298 K × lnK»
«lnK = =» 16.9 ✔
«K = e16.9 =» 2.19 × 107 ✔
Award [2] for correct final answer.
Accept range of 1.80 × 106–2.60 × 107.
If –43.5 is used then 4.25 × 107.
«Kc =» ✔
«shifts» left/towards reactants AND «forward reaction is» exothermic/ΔH is negative ✔